High-resolution structures of human Na v 1.7 reveal gating modulation through alpha-pi helical transition of S6 IV.
Huang, G., Liu, D., Wang, W., Wu, Q., Chen, J., Pan, X., Shen, H., Yan, N.(2022) Cell Rep 39: 110735-110735
- PubMed: 35476982
- DOI: https://doi.org/10.1016/j.celrep.2022.110735
- Primary Citation of Related Structures:
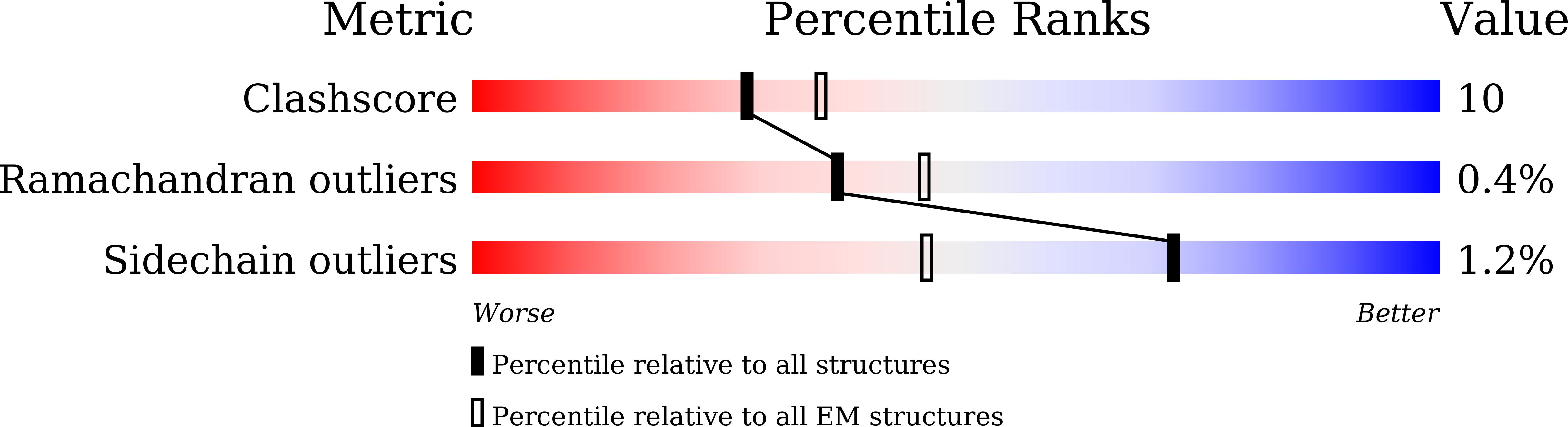
7W9K, 7W9L, 7W9M, 7W9P, 7W9T - PubMed Abstract:
Na v 1.7 represents a preeminent target for next-generation analgesics for its critical role in pain sensation. Here we report a 2.2-Å resolution cryo-EM structure of wild-type (WT) Na v 1.7 complexed with the β1 and β2 subunits that reveals several previously indiscernible cytosolic segments. Reprocessing of the cryo-EM data for our reported structures of Na v 1.7(E406K) bound to various toxins identifies two distinct conformations of S6 IV , one composed of α helical turns only and the other containing a π helical turn in the middle. The structure of ligand-free Na v 1.7(E406K), determined at 3.5-Å resolution, is identical to the WT channel, confirming that binding of Huwentoxin IV or Protoxin II to VSD II allosterically induces the α → π transition of S6 IV . The local secondary structural shift leads to contraction of the intracellular gate, closure of the fenestration on the interface of repeats I and IV, and rearrangement of the binding site for the fast inactivation motif.
Organizational Affiliation:
Westlake Laboratory of Life Sciences and Biomedicine, Key Laboratory of Structural Biology of Zhejiang Province, School of Life Sciences, Westlake University, 18 Shilongshan Road, Hangzhou, Zhejiang Province 310024, China; Institute of Biology, Westlake Institute for Advanced Study, 18 Shilongshan Road, Hangzhou, Zhejiang Province 310024, China.